Catalyst Testing for Automotive Emissions Reduction
The testing of different catalytic materials for automotive emissions reduction is a collaborative effort between Prof. Depcik and the Chemical and Petroleum Engineering Department (Dr. Susan Stagg-Williams). The system shown here consists of a quartz tube reaction chamber jacketed by a resistance heating furnace capable of achieving temperatures above 800ºC. The setup is capable of mixing multiple reaction gases, as well as changing mixing rates and overall flow rates in order to achieve a wide range of gas concentrations to more accurately represent engine exhaust conditions. Product concentrations are measured by gas chromotography (SRI 8610C) for CO2, H2, CO and other hydrocarbon components. NO, NO2, and total NOx are measured via chemilumenescence detection (CAI Laboratories HCLD 600 series). The reactor system is also capable of performing constant volume injections via a closed, loop six-way valve, which can be used to conduct adsorption and desorption studies. Currently, Lean NOx Trap (LNT) catalysts are being investigated in order to study the effect of catalyst degradation on LNT device performance. Specifically, the effect of noble metal sintering on lean phase NO oxidation is being evaluated in combination with catalyst simulation efforts to generate accurate and computationally efficient models for the implementation of LNT catalysts in lean combustion engine exhaust systems. The system is also being used to study catalyst degradation in novel cerium supported catalysts and their promoting properties for noble metal dispersion.
Representative paper: Experimental, Detailed, and Global Kinetic Reaction Model for NO Oxidation over Platinum/Alumina Catalysts - doi: 10.1007/s11144-015-0935-z

Experimental layout of the quartz tube reaction chamber and resistive heating furnace
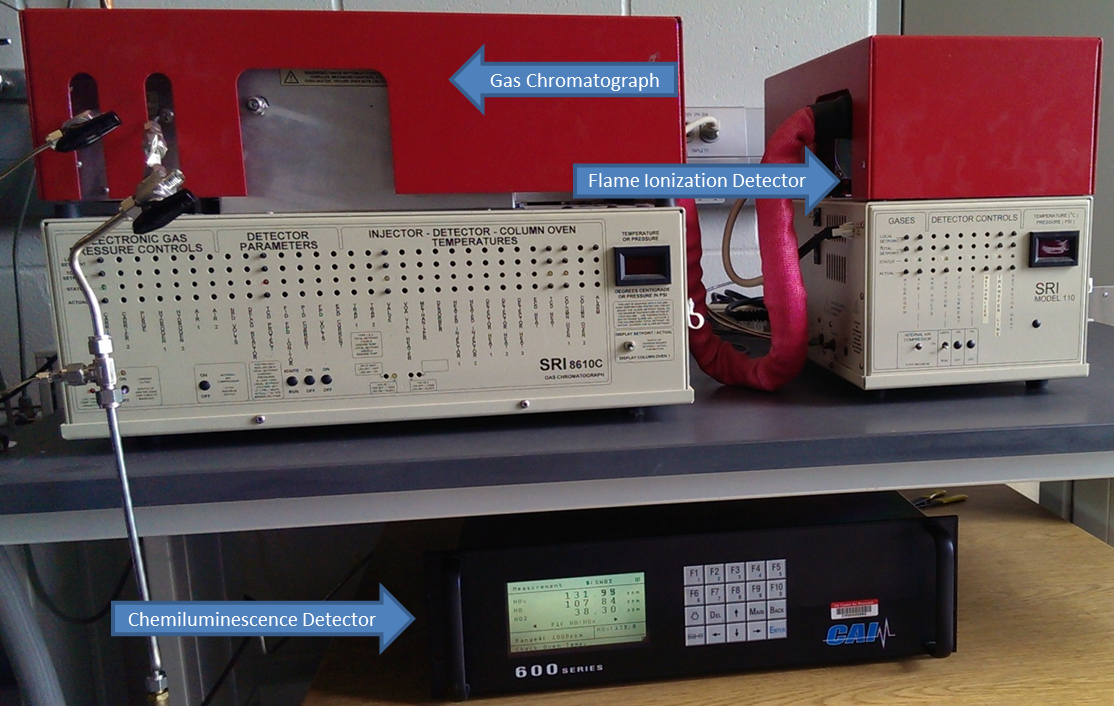
Species analyzers employed in data collection efforts
